PRODUCT NAME:
copper circle machine anode plate disc round continuous casting line
How to refine copper ingots
1. Copper ingot refers to the electrolytic purification of copper: crude copper (containing 99% copper) is pre made into thick plates as the anode, pure copper is made into thin plates as the cathode, and a mixture of sulfuric acid and copper sulfate is used as the electrolyte. After being electrified, copper dissolves from the anode into copper ions and moves towards the cathode. Upon reaching the cathode, electrons are obtained and pure copper (also known as electrolytic copper) is precipitated at the cathode.
2. Impurities in crude copper, such as iron and zinc, which are more active than copper, will dissolve with copper into ions (Zn and Fe). Due to the difficulty in precipitation of these ions compared to copper ions, adjusting the potential difference appropriately during electrolysis can avoid the precipitation of these ions on the anode. Impurities such as gold and silver, which are less active than copper, are deposited at the bottom of the electrolytic cell. The copper plate produced in this way is called "electrolytic copper" and has extremely high quality, which can be used to make electrical products. The sediment at the bottom of the electrolytic cell is called "anode mud", which is rich in gold and silver and is very valuable. It has extremely high economic value for extraction and processing.
PRODUCT PICTURES:
copper circle machine anode plate disc round continuous casting line

copper circle machine anode plate disc round continuous casting line

copper circle machine anode plate disc round continuous casting line

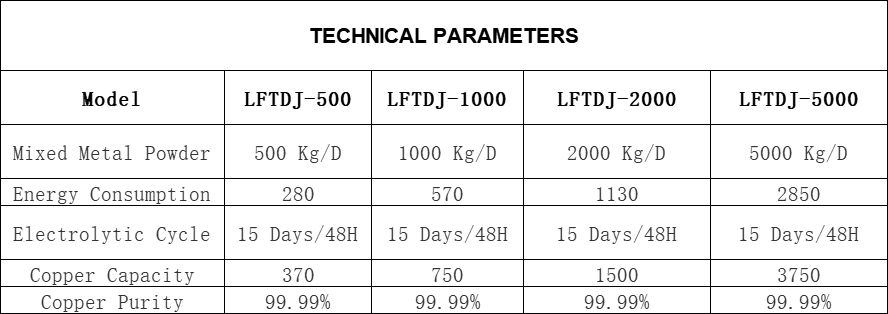
SPECIFIATIONS:
copper circle machine anode plate disc round continuous casting line
 English
English  Español
Español  Português
Português  русский
русский  français
français  日本語
日本語  Deutsch
Deutsch  Tiếng Việt
Tiếng Việt  Italiano
Italiano  Nederlands
Nederlands  ไทย
ไทย  Polski
Polski  한국어
한국어  Svenska
Svenska  Malay
Malay  বাংলা
বাংলা  हिन्दी
हिन्दी  Pilipino
Pilipino  Türk
Türk  عربى
عربى  Indonesia
Indonesia  norsk
norsk  čeština
čeština  Українська
Українська  Javanese
Javanese  فارسی
فارسی  తెలుగు
తెలుగు  Burmese
Burmese  български
български  Latine
Latine  Azərbaycan
Azərbaycan  Српски
Српски  Esperanto
Esperanto  Afrikaans
Afrikaans  Català
Català  Cymraeg
Cymraeg  Беларус
Беларус  Hrvatski
Hrvatski  Kreyòl ayisyen
Kreyòl ayisyen  Shqiptar
Shqiptar  Bosanski
Bosanski  Кыргыз тили
Кыргыз тили  ಕನ್ನಡ
ಕನ್ನಡ  IsiXhosa
IsiXhosa  Chichewa
Chichewa  Somali
Somali  O'zbek
O'zbek  հայերեն
հայերեն  Sundanese
Sundanese  Malagasy
Malagasy 
















